

The term electronegativity signifies the tendency of atom to attract the shared pair of electrons towards itself.
In compounds formed by bonding between two or more elements, one of the element have higher tendency to attract shared electrons. In comparison to this, down the group as the atomic size increases the pull of nucleus over outermost shell electrons decreases, so metallic character increases and nonmetallic character decreases.

The same is the reason why non metallic character increases from left to right. Due to which the tendency to lose electrons decreases i.e. In periods, from left to right, nuclear charge increases therefore the pull of nucleus over outermost shell becomes stronger. With increased nuclear charge nucleus tend to pull electrons closer to nucleus hence size of atom decreases.
However, in a period number of shells remains same but the nuclear charge (i.e. Down the group number of shells increases hence atomic size increases. the distance from center of nucleus to outermost shell. In groups even though atomic number increases by order 2-8-8-18-18-32 still the number of outermost shell electrons remains same and therefore valence remains same.Ītomic size refers to radius of an atom i.e. From left to right in periods, valence increases from 1 to 4 and then decreases to 0. Valency is the number of electrons needed to be accepted or donated so as to have a completely filled outermost shell. Some elements such as boron, silicon, germanium, arsenic show properties of both metal and nonmetals, these are called metalloids.
#PERIODIC TABLE CHEMISTRY THE CENTRAL SCIENCE SERIES#
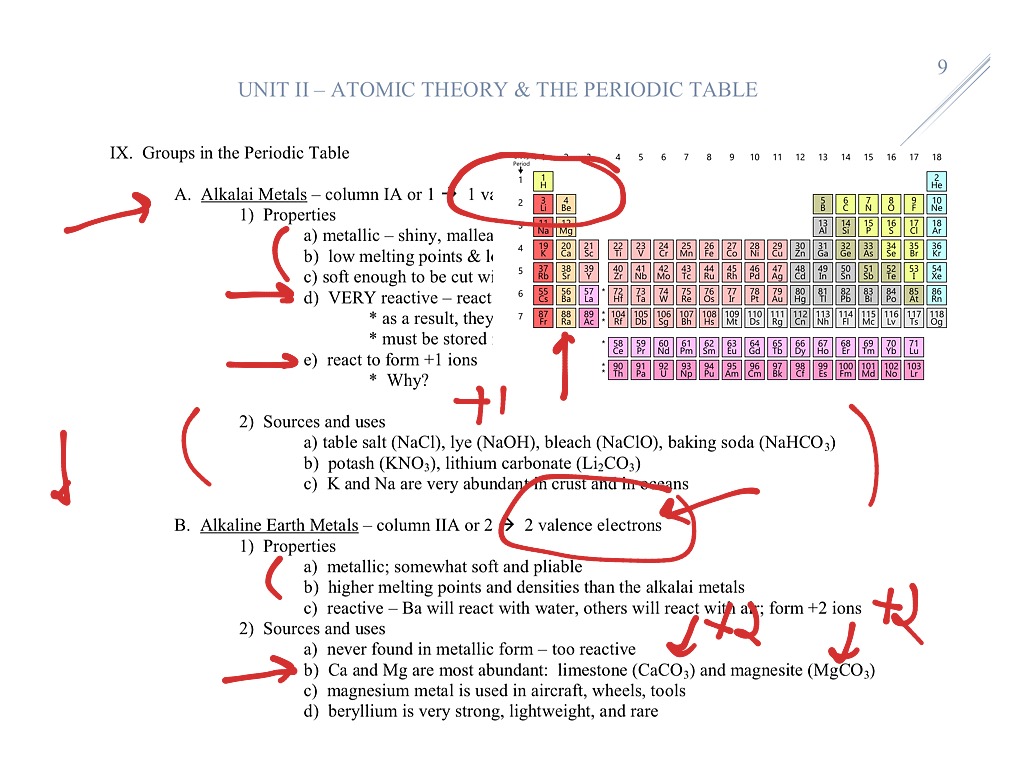
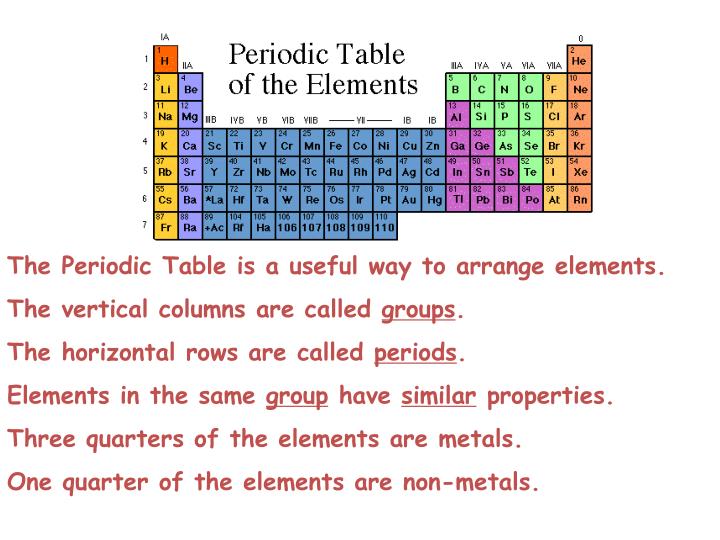
Actinides series start with actinium which is placed in group 3 period 7 since its properties are closely related to group 3 elements. Elements with atomic number between 90 to 103 are called as Actinides. It is placed in group 3 because of its similar properties with group 3 elements. Lanthanides series starts with Lanthanum which is placed in group 3 and period 6. Elements having atomic number between 58 to 71 are called Lanthanides. There are two series of these elements “Lanthanides” and “Actinides”. Elements placed at bottom of the periodic table are called as inner transition elements. These element contains two incomplete outermost shells. Their outermost shell is incomplete.Įlements of the groups from 3 to 12 are called as transition elements. Metals are arranged on left hand side of modern periodic table while non metals are arranged in right side.Įlements of the groups 1 and 2 and from 13 to 17 are called as normal elements. Noble gases has completely filled outermost shells. Group 17 has halogens and Group 18 has noble gases. Group 1 consists alkali metals, Group 2 contains alkaline earth metals. The seventh period is incomplete period.Įlements of same group have equal electrons in their outermost shell. Sixth period consists of 32 elements and called as the longest period. Fourth and fifth period has 18 elements so called as long periods. The second and third period contains 8 elements hence these are called short periods. The first period has only two elements hence it is the shortest period. Elements are placed based on their atomic number. There are 18 vertical columns known as ‘groups’ and 7 horizontal rows called ‘periods’ in the modern periodic table. Position of Elements in Modern Periodic Table


 0 kommentar(er)
0 kommentar(er)
